| Text |
Atherosclerosis
is a disease of the vessel wall involving lipid accumulation, chronic
inflammation, cell death, and thrombosis that causes heart disease and
stroke. Although elevated cholesterol levels are a recognized risk factor
for atherosclerosis, a growing number of studies suggest that oxidized
phospholipids may also play an important role in this condition.1,2 Phospholipids,
essential components of lipoproteins and cell membranes, are composed
of fatty acids bound to a glycerol backbone containing a polar head group.
They are susceptible to free-radical or enzymatic oxidation by myeloperoxidase,
lipoxygenase, and other enzymes that are present in the vessel wall. The
addition of oxygen to the polyunsaturated fatty acids produces prostaglandin-like
molecules, some of which then decompose and fragment to form additional
bioactive molecules.
Oxidized phospholipids accumulate under conditions of oxidative stress
during viral infections and in inflammatory conditions such as rheumatoid
arthritis and atherosclerosis; they are also generated in apoptotic and
necrotic cells.(1,2) Oxidized, but not native, phospholipids can interact
with specific receptors that mediate atherogenesis. In addition, oxidized
phospholipids contain reactive groups that can bind covalently to proteins,
forming lipid–protein adducts. These modified proteins become dysfunctional,
which can contribute to atherosclerosis. Phospholipid oxidation elicits
an immune response by creating new epitopes that are recognized by antibodies
of innate immunity, such as E06.(3) Thus, oxidized phospholipids are fundamentally
distinct from unoxidized phospholipids in their ability to interact with
cells, proteins, and the immune system in order to promote atherogenesis.
In vivo studies in human tissue have demonstrated the accumulation of
oxidized phospholipids in the vessel wall at all stages of atherosclerosis,
from early fatty streaks (in infants of mothers with hyperlipidemia) to
advanced complex lesions, suggesting that these lipids may contribute
to all stages of atherogenesis. In vitro studies and studies in which
oxidized phospholipids were injected into animals have demonstrated that
specific oxidized phospholipids can mediate many atherogenic processes
— from the earliest entry of monocytes into the vessel wall to thrombus
formation
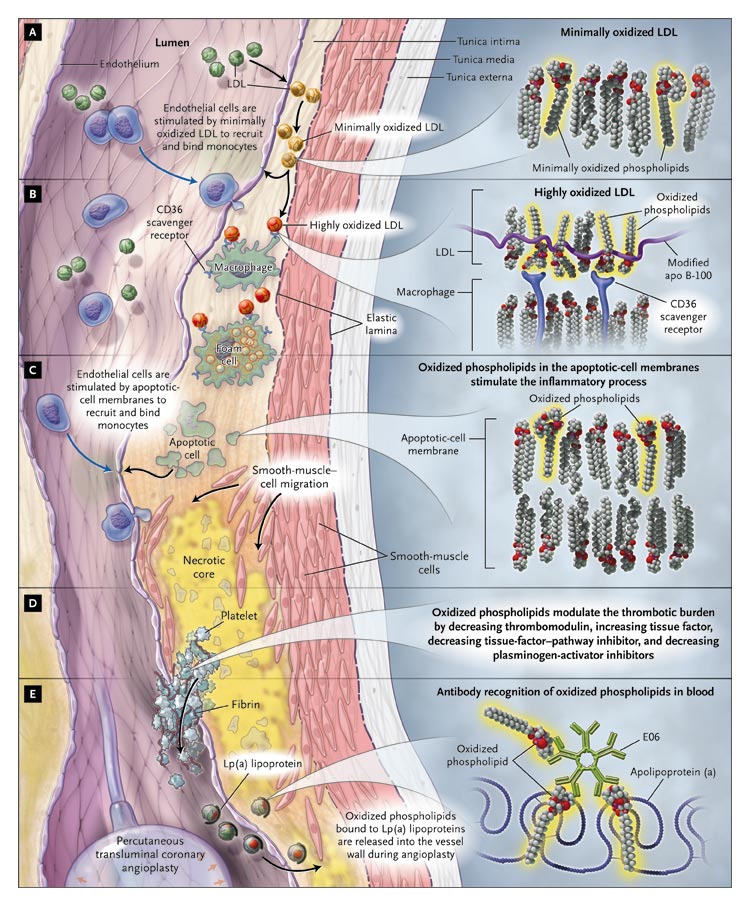
A Model
of the Roles of Oxidized Phospholipids in the Development of Atherosclerosis.
Low-density lipoprotein (LDL) moves into the subendothelial
space and becomes oxidized (Panel A). Inflammatory cells are recruited
into the vessel wall, take up the oxidized LDL through scavenger receptors,
and become foam cells (Panel B). The cell membranes of apoptotic cells
continue to recruit inflammatory cells into the vessel wall (Panel C).
Oxidized phospholipids also promote thrombosis through the modulation
of thrombotic factors (Panel D). Modified Lp(a) lipoprotein, which accumulates
in atherosclerotic lesions, can be detected at higher levels in the blood
after angioplasty with the use of E06, an antibody that recognizes oxidized
phospholipids (Panel E).
Oxidized
phospholipids activate the endothelium to bind monocytes (but not neutrophils)
and cause the endothelial cells and smooth-muscle cells to produce the
potent monocyte chemoattractant protein 1 and the differentiation factor
macrophage colony-stimulating factor. (Oxidized phospholipids can also
induce responses that protect cells from oxidative stress and inhibit
some acute, neutrophil-mediated inflammatory responses.(2))
In vivo, the presence of monocyte-binding molecules and chemotactic factors
causes monocytes to migrate into the subendothelial space and to differentiate
into macrophages. These macrophages can then release additional reactive
oxygen species, further oxidizing low-density lipoprotein to a form that
is recognized by scavenger receptors on macrophages and on smooth-muscle
cells; this uptake results in the formation of foam cells. Oxidized phospholipids,
either free or adducted to apolipoprotein B-100, are recognized by the
CD36 scavenger receptor.(4) Furthermore, these phospholipids bind to C-reactive
protein and could promote foam-cell formation through the Fcg
receptor.
As atherogenesis progresses (in response to cytokines produced by activated
endothelial cells and macrophages), smooth-muscle cells proliferate, enter
the intima, and form foam cells. Specific oxidized phospholipids at low
concentrations stimulate the proliferation of smooth-muscle cells. Ultimately,
the foam cells die by necrosis or apoptosis, and a necrotic core is formed.
At higher concentrations, oxidized phospholipids have been shown to regulate
smooth-muscle apoptosis by increasing the level of ceramide and facilitating
the release of cytochrome c from mitochondria. All the while, the inflammation
continues, with further entry of monocytes and lymphocytes into the vessel
wall. This continuing entry may be facilitated by oxidized phospholipids
that are present in the membranes of apoptotic and necrotic cells.
Ultimately, the plaque may rupture or erode, causing a thrombus to form.
Key enzymes in the coagulation pathway are also targets of oxidized phospholipids,
which increase the expression of tissue factor in endothelial cells, while
decreasing the expression of thrombomodulin and the activity of tissue-factor–pathway
inhibitor. Platelet activation is also stimulated by oxidized phospholipids.
Thus, oxidized phospholipids have proatherogenic effects on all vascular-wall
cells.
Although many of the studies cited above were performed in vitro, there
is growing evidence that oxidized phospholipids have a role in atherogenesis
in vivo. Knocking out or inhibiting receptors that recognize oxidized
phospholipids (including the platelet-activating–factor [PAF] receptor,
CD36, and toll-like receptors 2 and 4) leads to a decrease in experimental
atherosclerosis. Knocking out 12/15 lipoxygenase, an enzyme that oxidizes
polyunsaturated fatty acids, also results in decreased atherosclerosis.
Levels of myeloperoxidase, another oxidative enzyme, are correlated with
the risk of coronary artery disease. High-density lipoprotein (HDL) has
been shown to play a protective role in atherogenesis and alters the metabolism
of oxidized phospholipids. HDL contains proteins (such as apolipoprotein
A-I [apo A-I]) and enzymes (such as lecithin–cholesterol acyltransferase,
paraoxonase, and PAF–acetylhydrolase) that can prevent the formation
of oxidized phospholipids or destroy them once they have formed. Apo A-I
transfers the phospholipids to HDL for destruction. Knocking out paraoxonase
or PAF–acetylhydrolase increases atherosclerosis.
The same enzymes associated with HDL that destroy oxidized phospholipids
are also inhibited by them, creating a balance so that in the absence
of continued inflammation, HDL maintains enough functioning apo A-I and
enzyme activity to be antiinflammatory. During an acute-phase response
(e.g., after surgery) or during a chronic response (e.g., a chronic systemic
inflammation such as atherosclerosis), the balance can shift, and HDL
can become proinflammatory. In animal models of atherosclerosis, the balance
has been shifted back by the transgenic or adenovirus-mediated expression
of high concentrations of apo A-I or the exogenous administration of apo
A-I or apo A-I–mimetic peptides.(5)
The study by Tsimikas et al., reported in this issue of the Journal (pages
46–57), demonstrated a correlation between the levels of oxidized
phospholipids in the blood and levels of Lp(a) lipoprotein. The investigators
also determined that increased levels of Lp(a) lipoprotein and oxidized
phospholipids, in particles containing apolipoprotein B-100, correlated
with the risk of coronary artery disease and that combined hypercholesterolemia
plus increased levels of either oxidized phospholipids or Lp(a) lipoprotein
greatly increased the odds of coronary artery disease. Thus, this study
is the first to establish a causal connection between the levels of oxidized
phospholipids and the risk of coronary artery disease.
In summary, phospholipids are ubiquitous molecules that are important
to the structural integrity of cells and lipoproteins. When oxidized,
however, they can promote inflammation, are taken up by scavenger receptors
on macrophages, and are recognized by the innate immune system. Studies
suggest that proteins and enzymes that remove or destroy oxidized phospholipids
prevent atherosclerosis and that proteins and enzymes that produce or
retain oxidized phospholipids promote atherosclerosis. Thus, oxidized
phospholipids may be a diagnostic marker of coronary artery disease or
may represent a potential target for therapeutic intervention.
Source
Information: Dr. Berliner is a professor of medicine and pathology and
Dr. Watson is an assistant professor of medicine at the David Geffen School
of Medicine, University of California, Los Angeles.
References
1) Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence
for a role of phospholipid oxidation products in atherogenesis. Trends
Cardiovasc Med 2001;11:142-147.
2) Leitinger N. Oxidized phospholipids as modulators of inflammation in
atherosclerosis. Curr Opin Lipidol 2003;14:421-430.
3) Binder DJ. The role of natural antibodies in atherosclerosis. J Lipid
Res (in press).
4) Podrez EA. Identification of a novel family of oxidized phospholipids
that serve as ligands for the macrophage scavenger receptor CD 36. J Biol
Chem 2002;277:38503-38516.
5) Navab M, Ananthramaiah GM, Reddy ST, et al. The oxidation hypothesis
of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid
Res 2004;45:993-1007.
|
